Below is a full cross-post from Julie’s site http://fetalindustry.com
Julie Collorafi Jan 27
*For documentation on how Regeneron uses the HEK293T human embryonic kidney cell line, please see this article or this video.
Regeneron Used Human Fetal Liver To Humanize Their Mice
After an exhaustive search through Regeneron’s published studies, I have verified that Regeneron uses human fetal liver to engineer humanized mice capable of producing human antibodies. Human fetal liver obtained from aborted babies was used in several key studies to humanize the mice Regeneron uses to produce monoclonal antibodies—a fact that is not disclosed on the publicly available literature on the REGN-COV2 antibody cocktail.
The Gates Foundation-Funded MISTRG Mice

As it turns out, Regeneron has developed two humanized mice models, their gene-edited VelocImmune mice, and the MISTRG mice developed at Yale University from 2005 on, with funding from the Bill & Melinda Gates Foundation, which is humanized with fetal liver obtained from Advanced Bioscience Resources, the organ trafficking partner of Planned Parenthoods (exposed by David Daleiden’s undercover videos).
Yale University is a leader in stem cell research. Since 2007, Yale has maintained a Human Embryonic Stem Cell Core where human embryonic stem cells (hESCs) are stored and distributed, and new hESC lines and technology are being developed for researchers in the State of Connecticut. Yale has also been a leader in in vitro fertilization procedures, the first in the Northeast, since the 1980’s which could explain where Regeneron Pharmaceuticals obtains the embryos from IVF procedures which they acknowledged using in research and development in their April, 2020, press statement on stem cell use.

Dr. Richard Flavell, Professor of Immunobiology at Yale University Medical School, and an expert in transgenic mice, received a five-year $17 million grant from the Bill & Melinda Gates Foundation’s Grand Challenges in Global Health program to develop a human immune system mouse that could be used to test vaccines, so researchers could conduct in vivo testing that would not be safe to conduct in human trials.
Regeneron-Yale’s Close Collaboration on MISTRG Mice
Richard Flavell invited Regeneron Pharmaceuticals to collaborate on the project. Regeneron’s VelociGene technology was employed to knock-in four human cytokine genes in mice embyros to create a strain of immunodeficient mice that would tolerate and adapt to engrafted human hematopoietic (bone-marrow forming) cells.

This strain of chimeric mice (with human liver and blood cells in a human immune environment) was called the MSTRG mouse. The logo for the MSTRG mouse from the MSTRG Mice webpage at the Rongvaux Labs is shown on the left.
The MISTRG Mice webpage states that MISTRG mice were developed from 2005-2014 “in a collaborative effort between the labs of Pr. Richard Flavell (Yale University), Pr. Markus G. Manz (University of Zurich) and Regeneron Pharmaceuticals, with funding from the Bill and Melinda Gates Foundation.”
Another member of the MISTRG mice team mentioned on the MISTRG mice webpage is Cagan Gurer who is identified as an employee of Regeneron Pharmaceuticals and is apparently an expert in generating monoclonal antibodies. On his LinkedIn page he describes himself as an “R&D leader with 13 years of bio-pharmaceutical experience in generating monoclonal antibodies, bi-specifics, human T-cell receptors, and CARs for immuno-oncology therapies.”
(It’s important to note that George Yancopoulos and Sean Stevens of Regeneron, along with Richard Flavell and Markus Manz, are inventors listed on patents on genetically modified and engrafted human immune system mice.)
“Gain of Function Studies” on Mice Humanized with Human Fetal Liver

Dr. George Yancopoulos, Ph.D. and M.D., the co-founder and Chief Scientific Officer of Regeneron Pharmaceuticals, as a graduate student at Columbia University in 1985, proposed developing human immune system mice which could generate human antibodies.
Along with Dr. Richard Flavell and other members of the Yale MSTRG mouse team, Dr. Yancopoulos published two studies in 2011 with their new mouse model, which they called “hIL-3/GM-CSF KI mice”.
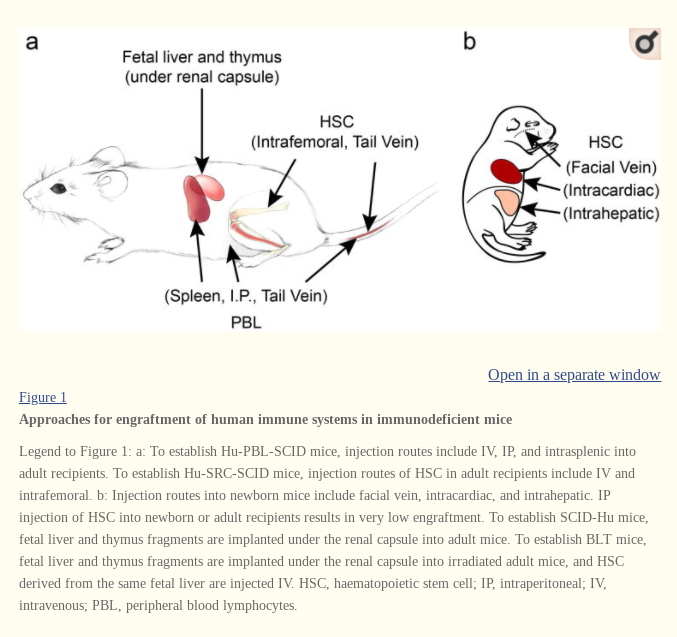
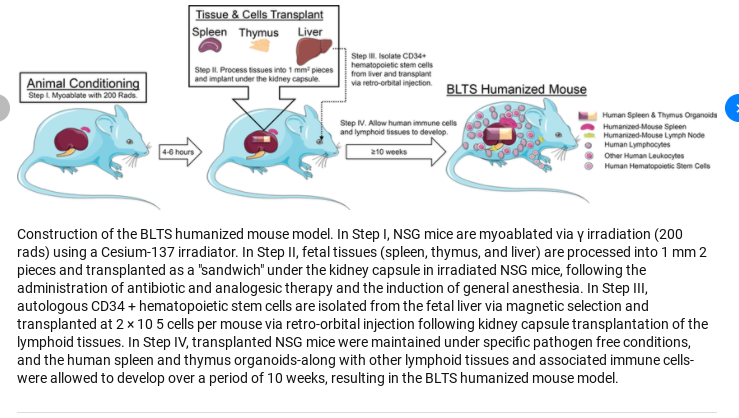
In the first study, published in February 8, 2011, they gene edited the mice embryos, and after birth, one set of mice were injected with CD34+ cells extracted from cord blood, and the other set with CD34+ cells extracted from human fetal liver, a technique invented by Professor Markus Manz, at the Institute of Research in Bellinzona, Switzerland. Samples of fetal liver were obtained from Albert Einstein Medical College, NY.
George Yancopoulos and Richard Flavell collaborated with other researchers on a second study published on September 15, 2011, with their human CSF-1 knockin mice. Using Velocigene technology, they inserted, or knocked in, a human CSF1 gene in the corresponding mouse gene in the mouse embryos. Human hematopoietic cells (CD34+ cells) extracted from human fetal liver (obtained from Advanced Bioscience Resources) were injected into the livers of the newborn mice.
The authors claimed evidence of enhanced amounts of human monocytes/macrophages in the bone marrow, spleens, peripheral blood, lungs and liver of the mice They also claimed augmented functional properties of the human monocytes/macrophages obtained from the humanized CSF-1 mice.
Interestingly, in the Discussion, the authors describe this study as a “gain of function” study and conclude that genetic manipulation of humanized mice models significantly improves the engraftment of human tissues onto mice.
In the Acknowledgments section, Advanced Bioscience Resources and Albert Einstein College of Medicine are thanked for their help in collecting human fetal liver samples. The authors also state that this study was funded by a grant from the Bill & Melinda Gates Foundation.
Regeneron-Yale’s New MI(S)TRG Mouse Model Produces Antibodies
A third study published on April 16, 2014, authored by Richard Flavell and other members of the Yale MISTRG mouse program, identifies the new knockin mouse strain as the MI(S)TRG mouse strain, describing how human versions of four cytokine genes were knocked into the corresponding genes of mouse embryos using Regeneron’s Velocigene technology. The same process of engrafting the livers of the newborn mice with CD34+ cells extracted from samples of human fetal liver was followed. The source of the human fetal liver was not disclosed.
A fourth study published on February 23, 2017, by George Yancopoulos, Richard Flavell and other members of the MISTRG mouse program, focused on antibody production in their mouse model, describing how they generated their new human IL-6 knockin mice. They point out how their mouse model does not require “complex surgery to transplant human fetal thymus and liver”, as is common industry praxis in the generation of BLT mice. Furthermore, their mice “can be used to exploit or evaluate immunization regimes that would be unethical or untenable in humans.”
The researchers followed the same process as described before: mouse embryos are gene-edited with Velocigene technology and, after birth, engrafted with human fetal liver-derived CD34+ cells. The fetal liver was obtained from Albert Einstein of Medicine and Advanced Bioscience Resources.
The authors conclude that there was enhanced antibody production in their humanized mouse model.
A fifth study. also co-authored by George Yancopoulos, Richard Flavell, and others was published on November, 7, 2017. The generation of knockin mice was performed with Regeneron’s Velocigene technology, and CD34+ cells extracted from human fetal liver were injected into the livers of the newborn mice. The source of the human fetal liver is not disclosed. In this study, the authors note that their humanized mouse model supports the development and function of NK (natural killer) cells and lymphocytes.
MISTRG6-hACE2 COVID-19 Mouse Used To Test Monoclonal Antibodies
On March 17, 2021, a sixth study was published by Richard Flavell and other members of the MISTRG mouse team, in which they rolled out the MISTRG6-hACE2 model, adapted for COVID-19 research, specifically, the testing of monoclonal antibody therapy.
The same process of generating knockin mice with Regeneron’s Velocigene technology is described, followed by the engraftment of human CD34+ cells from human fetal liver in the livers of newborn mice. The source of the human fetal liver is not disclosed.
In the Acknowledgments section, it is noted that the study was conducted in a Regeneron lab, and the Yale researchers were working under a material transfer agreement with Regeneron.
The study’s purpose was to infect the MISTRG6-hACE2 mouse model with SARS-CoV2 and test the efficacy of monoclonal antibody therapy. The authors concluded that the MISTRG-hACE2 humanized mouse could be of value in determining the timing and efficacy of monoclonal antibody treatments.
Human Fetal Liver Required for Complete Humanization of MISTRG Mice
It is evident from the series of published studies by Regeneron and Yale researchers that the knockin process of inserting human cytokine genes on corresponding genes of mouse embryos resulted in a new strain of mice that support the engraftment of human hepatopoietic cells from fetal, newborn or adult origin.
Furthermore, six of their published studies demonstrate that fetal liver obtained from aborted babies is the preferred choice for engraftment and is the final step of humanization required for the production of antibodies and the testing of monoclonal antibody treatments—a step in the process that has never been disclosed in Regeneron’s online literature about their monoclonal antibody cocktail.
Dr. Tara Sander Lee, of Charlotte Lozier Institute, in her statements, only described the initial Velocigene gene-editing process and is apparently unaware of Regeneron’s long published history of using human fetal liver as a necessary step in the humanization of their mice.
As we have seen, George Yancopoulos’ lifetime goal ever since the 1980’s was to make humanized mice capable of generating fully human monoclonal antibodies, and the MISTRG mice seem to be the fulfillment of that dream. As the MISTRG Mice website explains. the fetal liver-engrafted MISTRG mice support the development of monocytes, macrophages and lymphocytes, and also produce IL-15 which supports the development of human NK cells, all necessary for the production of antibodies.
“The Most Valuable Mice Ever Created”

Dr. George Yancopoulos calls his VelocImmune mice “the most valuable mice ever created” because they have generated billions of dollars in revenue and have led to several FDA-approved drugs. It is not clear, however, where the work of the seemingly innocuous genetically engineered VelocImmune mice ends, and the work of the gruesome MISTRG mice, each garnished with a hefty dose of human fetal liver, begins.
Is Regeneron’s close involvement in the development of the Gates Foundation-funded MISTRG gain-of-function humanized mouse project merely an extraneous academic exercise? This doesn’t seem likely since the engraftment of aborted baby liver is required for the production of fully humanized monoclonal antibodies and for the testing of the monoclonal antibody cocktail.
“Conservative” COVID-19 Treatment of Choice
Dr. Tara Sander Lee’s well-intentioned attempt to sanitize Regeneron’s monoclonal antibody therapy from any abortion-tainted association is unsustainable, but it has provided needed cover for Regeneron to establish its monoclonal antibody cocktail as the “conservative” treatment of choice. A NY Times article wryly commented on how Regeneron’s cocktail has met with “near- universal acceptance” by “vaccine resisters”, mainstream doctors and conservative radio hosts alike and reports how conservative Republican governors have embraced it as an antidote.
However, as we have seen, in addition to the use of the HEK293 stem cell line in the production of REGN-COV2, it is very likely, given Regeneron’s well-documented published research, that “fresh and never frozen” human fetal liver harvested from still-living aborted babies was used to humanize the mice which made the monoclonal antibodies casirivimab and imdevimab, thus radically increasing the amount of cannibalized fetal material being infused into each patient.
Regeneron Pharmaceuticals needs to come clean about the exact role of the fetal liver-engrafted MISTRG mice in the production of its monoclonal antibody therapy, and the Charlotte Lozier Institute needs to reassess their insistence that no fetal tissue was used in the production of REGN-COV2, or in their humanized mice.
Regeneron’s degenerate COVID-19 medicine deserves much more scrutiny. It has been given a free pass for far too long.







 Julie Collorafi
Julie Collorafi